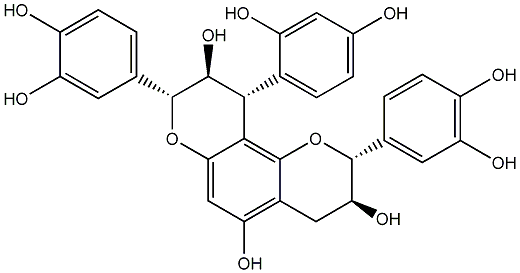
| Name |
(10R)-8,9-Dihydro-10-(2,4-dihydroxyphenyl)-8-(3,4-dihydroxyphenyl)-9-hydroxy-10H-pyrano[2,3-h]catechin |
| Formula |
C30H26O11 |
| Mw |
562.14751167 |
| CAS RN |
119067-90-2 |
| C_ID |
C00008947
, 
|
| InChIKey |
AMVCEZYBLCNDSF-JVJSVYAENA-N |
| InChICode |
InChI=1S/C30H26O11/c31-14-3-4-15(19(34)9-14)25-26-24(40-29(27(25)39)13-2-6-18(33)22(37)8-13)11-20(35)16-10-23(38)28(41-30(16)26)12-1-5-17(32)21(36)7-12/h1-9,11,23,25,27-29,31-39H,10H2/t23-,25-,27+,28+,29-/m0/s1 |
| SMILES |
Oc1ccc([C@@H]2c3c(cc(O)c4c3O[C@H](c3ccc(O)c(O)c3)[C@@H](O)C4)O[C@H](c3ccc(O)c(O)c3)[C@H]2O)c(O)c1 |
| Start Substs in Alk. Biosynthesis (Prediction) |
|
| Organism |
| Kingdom |
Family |
Species |
Reference |
| Plantae | Fabaceae | Guibourtia coleosperma  | Ref. |
|
|
zoom in
| Organism | Guibourtia coleosperma |
|---|
| Reference | Harborne, The Handbook of Natural Flavonoids, 2, (1999), 355,Flavans and proanthocyanidins
Steynberg,J.Chem.Soc.,Chem.Commun,(1988),1055
Steynberg,J.Chem.Soc.Perkin Trans.,1,(1988),3331 |
|---|
|